FDA Unveils Transformative Guidance for Computational Modeling in Medical Device Submissions

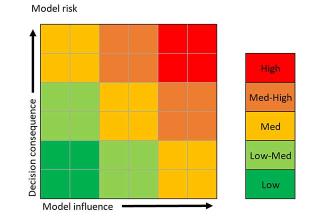
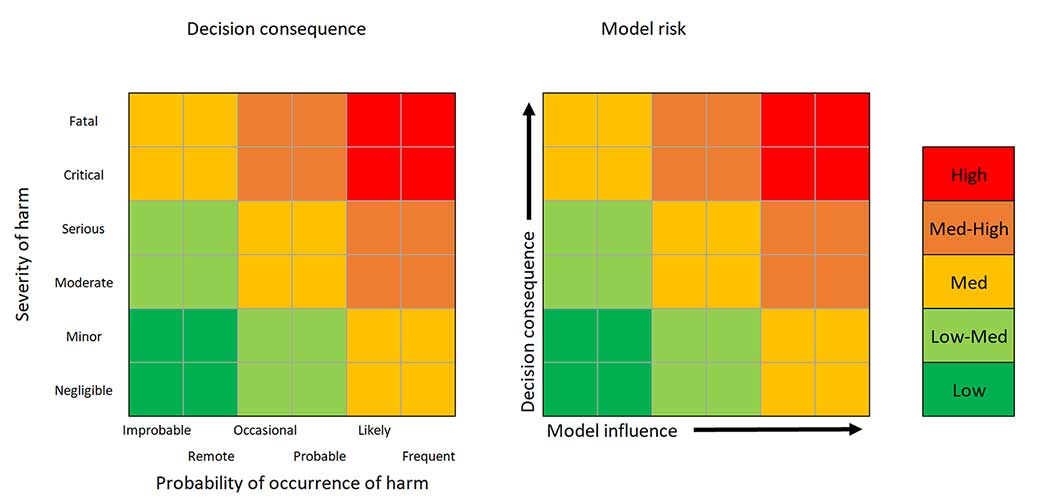 Part of the computational model risk assessment workflow used for an overall model credibility assessment.
Part of the computational model risk assessment workflow used for an overall model credibility assessment.
In late 2023, the Food and Drug Administration’s Center for Devices and Radiological Health (CDRH) released a guidance document that will have a profound effect on the use of computational modeling and simulation for medical device submissions. The first draft of “Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions: Guidance for Industry and Food and Drug Administration Staff” was released in December 2021, along with a call for comments from industry and affected stakeholders. This input was collected and incorporated, resulting in the final version released on November 16, 2023.
This CDRH guidance document is applicable to first-principles models (i.e., physics-based or mechanistic models) that either stand alone or are part of a hybrid computational method. Fundamentally, the guidance bases itself on ASME V&V 40-2018 to create a framework for the risk-informed credibility assessment of the computational models used in the regulatory submission process. Given the risk-informed approach, the guidance aligns with those typically used within the quality management systems of medical device manufacturers. As a result, the requirements on physical test and computational model data are now better aligned, making it more convenient and efficient to use both evidence streams in regulatory submissions, as well as streamlining the communication and acceptance of computational evidence. Examples of submission content covered by this guidance are the fatigue durability assessment of an implantable Class III device using finite element analysis, and hemodynamic-induced loading and thrombosis assessments using computational fluid dynamics. Both examples are typical of the work and services that our life sciences group regularly provides to its clients. Our client services span the whole product life cycle from novel concept characterization and design validation assessments to regulatory submission support and post-market population predictions.
Having used the draft version of the guidance document in the Dassault Systèmes/FDA ISCT (In Silico Clinical Trial) Enrichment Project, where I was credibility team lead, I found it to be invaluable. For those unfamiliar with the ISCT enrichment project, it is a collaboration investigating the use of various computational models and tools in a virtual ISCT of a generic edge-to-edge mitral valve repair. The framework laid out in the guidance document formed the basis of the credibility assessment that our team completed. For this project, I found the guidance document was easy to follow, aligns well with good quality management processes, and most importantly, provides a common language to discuss the merits of the proposed physics-based modeling approaches with the FDA.
It should also be noted that this guidance document does not provide a checklist and is not prescriptive, rather it is a general framework that can be adapted to fit your specific situation. Our team has applied the framework in this guidance document for our clients to support their regulatory needs. If you would like help navigating this and other guidance documents, our expert team is here to assist you.
Given the industry buzz that it has generated, we are watching for other similar guidance documents related to embedded software and AI/ML models to come out soon.