
Biomedical Digital Twins
Design and review of new medical technology, accelerated with digital twin modeling.
The biomedical industry is looking to innovative methods, such as in silico trials, to save time and resources and reduce clinical-trial risks. Combining machine learning with our engineering simulation expertise, we’re creating virtual models of living systems that can serve as a substitute for physical testing. In the U.S. market, this approach can aid both designers of new technology and the Food and Drug Administration (FDA) in evaluating those technologies.
Our team produced an in silico simulation, or digital twin, of a surgical implant of a generic stent graft for a virtual patient with a thoracic aortic aneurysm. They used machine learning to analyze how the patient’s anatomy and the structure of the stent graft affected therapeutic outcomes. The simulation identified the inputs that will have the most influence on the likelihood of the stent migrating or leaking.
“Efficacy and safety analyses like this are critical for the efficient development of life-saving therapies,” says Kristian Debus, head of our life sciences team. “Through rapid iterations, developers can quickly zero in on optimal design strategies."
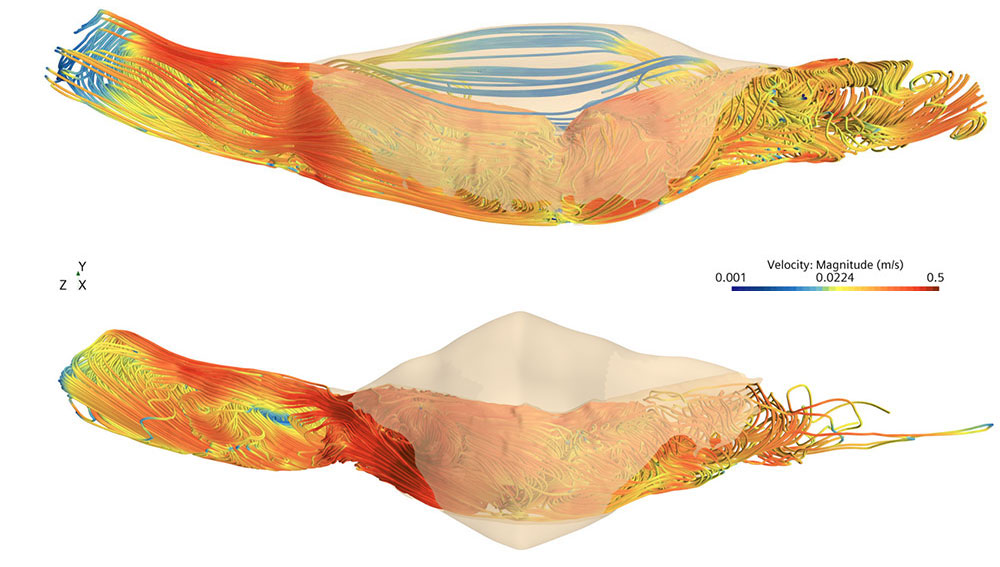
Stent grafts are often used to treat aneurysms by reinforcing a weakened vessel wall. Here, we used computational fluid dynamics modeling to simulate blood flow through and around a stent graft (top), in which red denotes the highest velocity and blue denotes the lowest. The bulge above the stent shows leakage. The bottom image shows a successful stent placement without leakage, in which the aneurysm is depicted as the tan, shaded bulge.
Our next challenge? Based on this demonstrator case, we’re now developing a graphical framework that will facilitate discussions between a medical device maker and the FDA. The framework will be the first to combine real-world clinical outcomes and simulation data in this format to maximize the benefits of digital twins in device design.










